-
Phase 1
Randomized, placebo-controlled safety study with dose escalation
- Found 12 µg to be well-tolerated
Activation of immune system evident: Induction of cytokines & chemokines (IFN-γ, IL-18, CCL2, CCL4, CXCL9, CXCL10, CXCL11)
Stimulation of progenitor cells: Increased lymphocytes, neutrophils, platelets & reticulocytes
32 Healthy Subjects (21 HemaMax-Treated) - Completed
-
Phase 1b
Expanded, single dose (12 µg), randomized, placebo-controlled study in healthy subjects
60 Healthy Subjects (48 HemaMax-Treated) - Completed
-
Phase 2
Randomized, placebo-controlled study in healthy subjects representative of the U.S. population in age, gender & BMI
No immunogenicity up to day 45, after a single dose
200 Healthy Subjects (160 HemaMax-Treated) - Completed
-
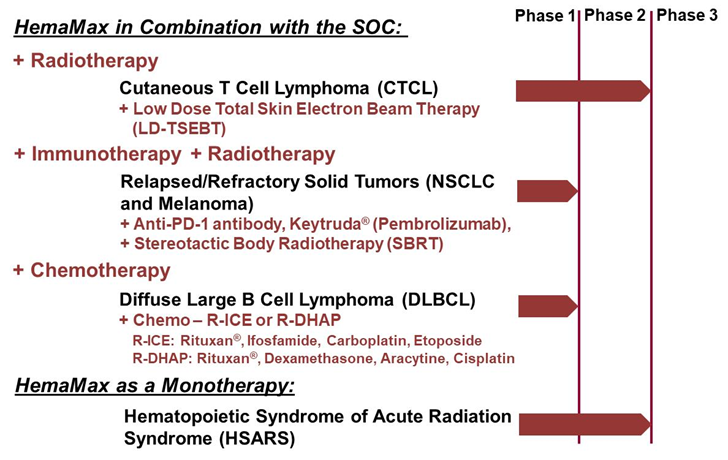
Phase 2
Single-arm study in Cutaneous T Cell Lymphoma (CTCL)
Treatment in combination with low dose, total skin electron beam radiation (LD-TSEBT)
Endpoints: Complete response & time to treatment failure compared to historical data for LD-TSEBT
No immunogenicity up to week 47, after repeat dosing
16 CTCL Patients
HemaMax-Treated
Ongoing